Research
The Hayashi research group is developing thermochemical and/or catalytic conversion systems of solid carbon resources such as coal and biomass
Project examples
- Development of reactors and reaction control methods for low temperature fast gasification
- Modeling and kinetic analysis of char gasification
- Green conversion of biomass to chemicals
- High strengh metallurgical coke from low-grade carbon resources
- Carbon neutral/negative system for power and chemicals coproduction
- Automated reactor with dynamic and presice control
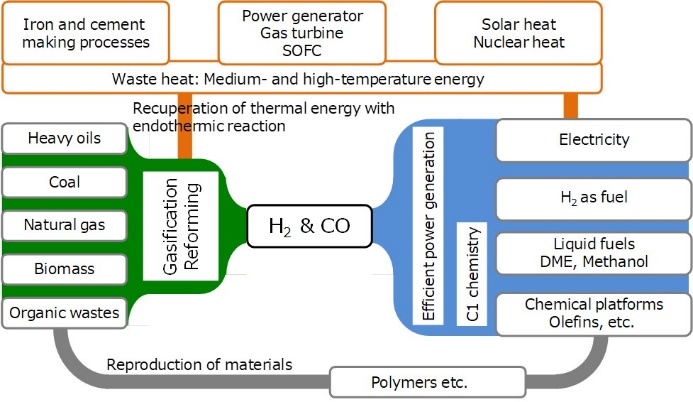
Reactors and reaction control methods for low temperature fast gasification of coal and biomass
Gasification is a technology that combines thermal energy with chemical energy of carbon resources to produce chemical energy as a form of syngas. The major scientific challenge is how to minimize the loss of chemical energy in the gasification, which requires decrease in the temperature of gasification as low as possible. We have developed the low temperature gasification system based on some important concepts; “separation of pyrolysis from gasification and reforming”, “intensification and elimination of volatiles-char interaction(VCI)”, “chemical recuperation of thermal energy”, “potassium catalyst migrating between gas phase and char surface”. The system enables complete gasification of biomass and lignite at temperatures as low as 700-800℃ with nearly complete removal of tar.
Adv Powder Technol 31 (2020) 867, Applied Energy 233-234 (2019) 156, Energy Fuels 30 (2016) 1616, Energy Fuels 28 (2014) 6407, Energy Fuels 28 (2013) 4, Energy Fuels 26 (2012) 199
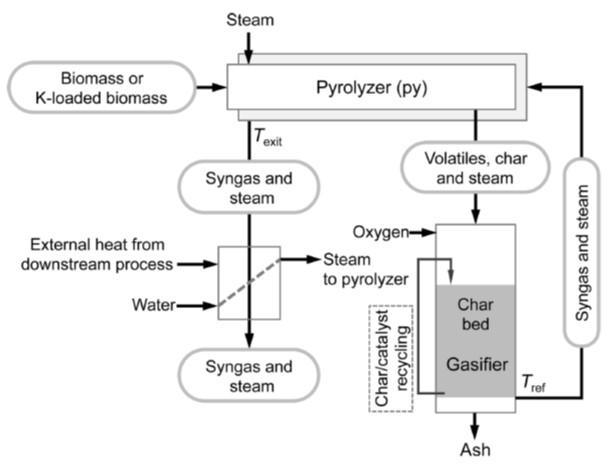
Modeling and kinetic analysis of char gasification
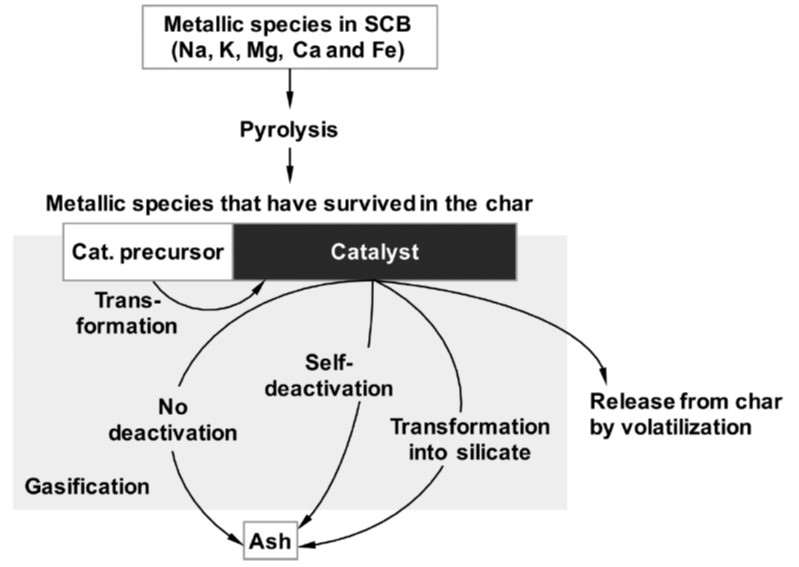
We have recognized the difficulty of representing kinetics of biomass and lignite chars gasification with conventional gasification models based on surface areas because the reaction rate is controlled dominantly by catalysis of ash metallic species. We are studying a new kinetic model which assumes that non-catalytic gasification (first order reaction) and catalytic gasification (zero-th order reaction) progress in parallel. The model represents kinetics of gasification from diverse resources very well in the conversion range from 0 to over 99.9%. The kinetics analysis also reveals complicated mechanism of catalysis in the gasification
Energy Fuels 34 (2020) 225, Energy Fuels 33 (2019) 5996, Energy Fuels 32 (2018) 4255, Energy Fuels 30 (2016) 1636, Energy Fuels 28 (2014) 6407
Green conversion of biomass to chemicals
Chemical products nowadays are mostly produced from fossil fuels. Depletion fossil fuels in future necessitates a development of chemical industry using only an alternative feedstock, biomass. We study thermochemical methods such as pyrolysis and hydrothermal conversion for converting biomass to valuable chemicals in an environmentally friendly manner. An example is pyrolysis-based conversion of cellulose. Pyrolysis is unique amongst cellulose depolymerization methods since it does not need any solvent and other chemical reagents, and the reaction rate is very high compared to enzymatic saccharification methods. We have succeeded in reforming the pyrolysis volatiles to levoglucosenone in high yields, which is further converted to bulk and fine chemicals. In a study on lignin, we have found that oxidative pretreatment with hydrogen peroxide is very effective for depolymerization of lignin to yield its monomers with hydrogenolysis over nickel catalyst.
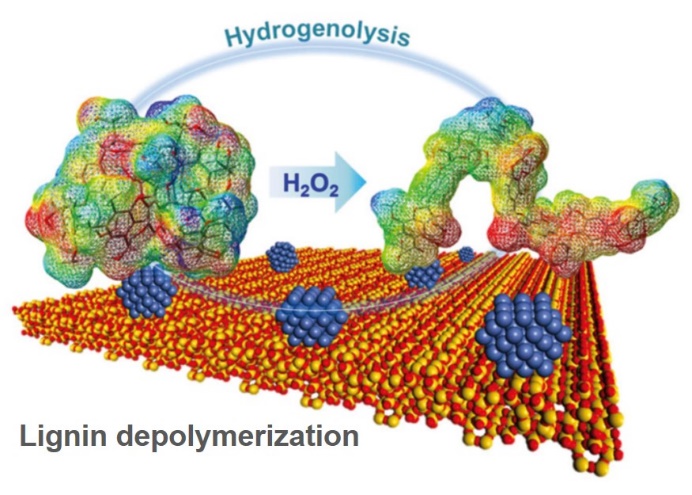
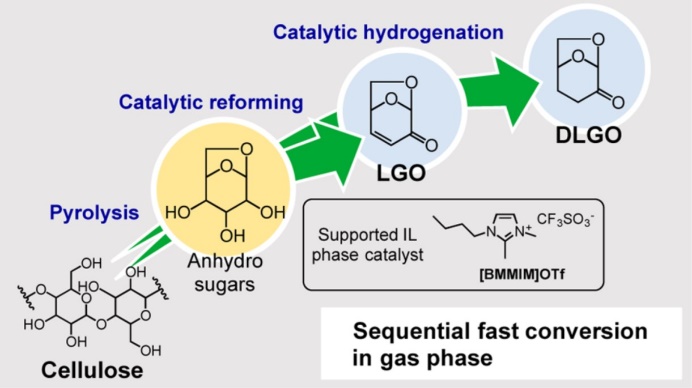
Energy Fuels 34 (2020) 7183, ACS Sustainable Chem Eng 7 (2019) 5892, Eur J Org Chem (2018) 2028, Green Chem 19 (2017) 2636, ACS Sustainable Chem Eng 5 (2017) 1132, Energy Fuels 28 (2014) 76
High strength metallurgical coke from low-grade carbon resources

An important challenge of iron making industry is a sharp increase in demand and the resulting price increase and depletion of coking coals. Our technology, which is a sequence of hot-briquetting and carbonization, enables production of high strength coke from non-coking coals such as lignite or even from biomass. We are currently studying on this technology to be industrially feasible with several approaches; understanding mechanisms of high strength coke formation, improvement of coke strength with feedstock pretreatment, diversifying types of feedstock, application as a high-value material, and so on.
ISIJ Int 59 (2019) 1440, ISIJ Int 59 (2019) 1449, Energy Fuels 32 (2018) 4364, ISIJ Int 55 (2015) 765, ISIJ Int 54 (2014) 2461, Energy Fuels 27 (2013) 6607, Energy Fuels 26 (2012) 296
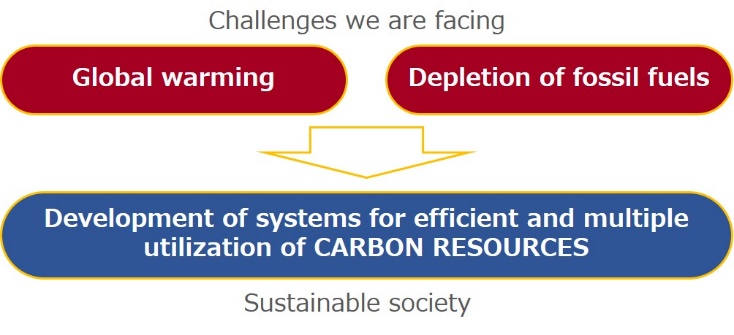
Carbon neutral/negative system for power and chemicals coproduction
Japan has stated reduction of CO2 emission to a level 80% below the current amount in 2050. Toward this difficult goal, and as a method for realizing sustainable carbon cycle society, we have proposed a process that co-produces power and chemicals from carbon resources (fossil fuels and biomass) while having carbon neutral or negative nature. Researches on the evaluation of process performance in terms of impact on CO2 mitigation, process simulation, and development of elemental technologies are in progress.
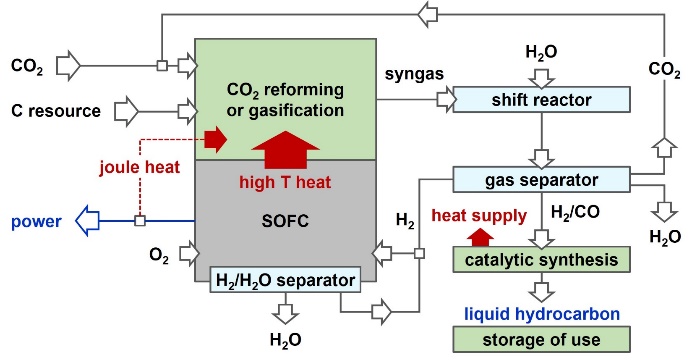
Automated reactor system with dynamic and precise control
We aim to apply recent advanced sensing equipment and IoT technologies for chemical synthesis. The dynamic and precise operation of the chemical process is one of the goals for chemical engineering in the 21st century. An automated chemical experiment is also an important target because it will dramatically improve productivity and reproducibility of chemistry research. Microreactors are also included in our study scope as an important enabling tool.
Scientific Reports 10 (2020) 7685, Langmuir 35 (2019) 2236, Org Proc Res Dev 23 (2019) 807


